News overview
Sorbent technology for sustainable dialysis
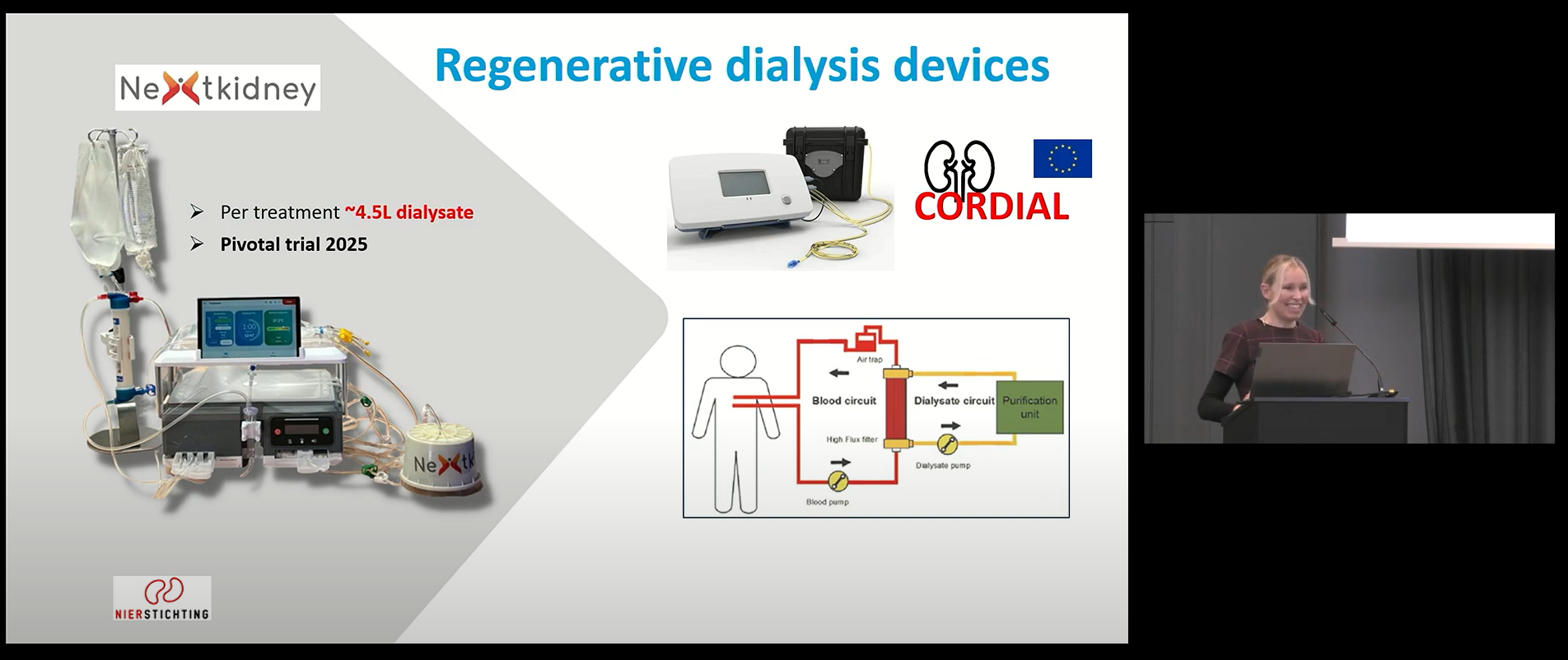
The WEAKID sorbent technology could be interesting to reduce dialysis water consumption. Last week, Karin Gerritsen presented innovative ideas in dialysis on the KDIGO Controversies Conference on Green Dialysis in Berlin.
Read moreThe dialysis users are the ultimate experts

Direct involvement of dialysis users is essential for the succes of this project and crucial for the development of the WEAKID device! Therefore we appreciate that already 65 surveys have been completed and we expect to reach our goal of 100 this summer. We will also soon plan the interactive focus groups: the first one will take place in the Netherlands, with similar sessions later in Spain and Italy.
Read moreThe FIH trial continues in Madrid with the 6th participant
In February 2025, the 6th participant in the First-in-Human trial will be included at the Hospital Universitario la Paz, in Madrid.
Read moreThe FIH trial continues in Modena with the 5th participant
In September 2024, the 5th participant in the First-in-Human trial will be included at the University Hospital of Modena.
Read moreApproval to start FIH trial in Modena and Madrid

The sites in Modena (Italy) and Madrid (Spain) have received approval to execute the First-in-Human CORDIAL trial.
Read moreCORDIAL survey in English, Dutch, Spanish and Italian
The CORDIAL survey for patients to investigate the preferences for new peritoneal dialysis devices is now available in English, Dutch, Spanish and Italian.
Read moreCORDIAL survey available in English and Dutch

The CORDIAL survey for patients to investigate the preferences for new peritoneal dialysis devices is now available in English and Dutch.
Read moreProject period extended with six months
The project CORDIAL has been extended from 3 years to 3.5 years. The current new end date is 31 December 2024. A second extension will be evaluated after summer 2024.
Read more

